Abstract
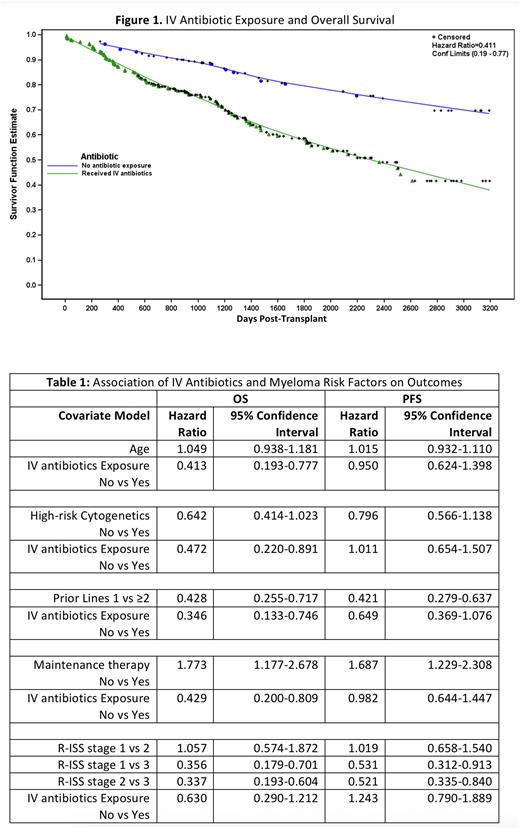
Autologous Hematopoietic stem cell transplantation (HSCT) following high-dose chemotherapy is an integral part of multiple myeloma (MM) treatment. While many factors impact HSCT success, low gut microbiome diversity has been shown to correlate with worse outcomes post-transplant (Khan et al, Blood 2021). HSCT patients receive intravenous (IV) antibiotics in response to neutropenic fever or bacterial infections, which can drive a lower microbiome diversity post-transplant (El Jurdi et al, BBMT 2018). Given the link between antibiotic exposure, low microbial diversity, and outcomes, we hypothesized that antibiotic exposure during transplant may adversely affect survival. To test this hypothesis, we performed a single-center retrospective study of transplant outcomes and IV antibiotic exposure at the University of Wisconsin from 2010-2017. Two hundred eighty-six patients underwent a first autologous HSCT using melphalan conditioning. All subjects received levofloxacin prophylaxis. Two hundred thirty-seven subjects received IV antibiotics including cefepime or piperacillin/tazobactam and vancomycin predominantly to treat neutropenic fever. Forty-nine patients did not require IV antibiotics. Patient demographics and disease factors were fairly balanced between the IV antibiotic and no antibiotic group, including age, sex, body mass index (BMI), presence of high-risk cytogenetics, number of prior lines of therapy, and use of maintenance therapy post-transplant. There was a higher proportion of Revised International Staging System (R-ISS) stage 1 myeloma in the no antibiotic cohort (12/39, 31%) versus the antibiotic cohort (37/179, 21%) but this was not statistically significant (p = 0.28). We observed an adverse effect of antibiotic exposure on overall survival (OS) (Figure 1, hazard ratio (HR) 0.41 IV antibiotic vs no antibiotic, 95% confidence interval (CI) 0.19 - 0.77), but not progression-free survival (PFS) (HR 0.95, 95% CI 0.62 - 1.4). Greater number of prior lines of therapy, higher R-ISS stage, and the absence of maintenance therapy each observed an adverse effect on OS when adjusting for IV antibiotic exposure (Table 1). On univariate analysis, high risk cytogenetics such as t(4;14), del17p, and t(14;16) was associated with inferior OS (HR no vs yes 0.625, 95% CI 0.40-0.99, p=0.041) but lost statistical significance when modelled together with IV antibiotic exposure HR no vs. yes 0.64, 95% CI 0.41-1.02), presumably due to sample size. After adjusting for age, presence of high-risk cytogenetics, number of prior lines, and use of maintenance therapy, the adverse effect of antibiotics maintained its statistically significant impact (Table 1). When adjusting for R-ISS stage, a similar adverse effect was noted but this was no longer statistically significant, possibly due to low numbers of reported R-ISS stage 3 in the no-antibiotic cohort (n=6). We conclude that IV antibiotic exposure confers an adverse effect on overall survival post-transplant. Inspection of the survival curve from the proportional hazards model (Figure 1) suggests this is unrelated to immediate post-transplant infectious complications. This finding supports a growing body of literature describing adverse long-term clinical impact of antibiotic exposure and on outcomes in cellular therapy, which we hypothesize is related to deleterious effects of antibiotic exposure on the gut microbiome. Larger prospective studies are necessary to further examine this relationship on myeloma outcomes.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal